Osmosis
Osmosis is a pressure-driven mechanical process, not merely the random diffusion of molecules.
Osmosis is a pressure-driven mechanical process, not merely the random diffusion of molecules.
While often simplified as "water moving to where there is less of it," osmosis is actually a thermodynamic interaction where heat from the surroundings is converted into mechanical energy. Conclusive research has refuted the idea that osmosis is caused by the "dilution" of water or the "attraction" of solutes. Instead, it is a pressure-driven flow: the presence of a solute reduces the pressure that water molecules exert on each other, allowing external pure water to "force" its way into the solution.
This force is a "colligative property," meaning the intensity of the pressure depends entirely on the concentration of the solute particles, not their chemical identity. Whether you dissolve salt, sugar, or proteins, the osmotic pressure remains the same as long as the number of particles is equal. This allows the process to be predictable across vastly different chemical environments, from industrial vats to human blood.
Cellular survival depends on the delicate maintenance of turgor pressure against semipermeable membranes.
Cellular survival depends on the delicate maintenance of turgor pressure against semipermeable membranes.
Osmosis is the primary engine for transporting water into and out of living cells. Because biological membranes are picky—allowing small molecules like water and oxygen through while blocking larger ions and proteins—cells exist in a constant state of osmotic tension. In plants, this creates "turgor pressure," the internal force that pushes the cell membrane against the cell wall. Without this osmotic intake, plants lose their structural integrity, becoming flaccid or "plasmolyzed."
In animals, the balance is even more precarious because animal cells lack rigid walls. If a cell is placed in a hypotonic environment (too much fresh water), it will swell and potentially burst. Conversely, in a hypertonic environment (too much salt), it shrivels. This explains why freshwater fish die in the ocean and why salt is lethal to soft-bodied organisms like slugs; the salt literally pulls the water out of their cells until they collapse.
The study of "push" evolved from ancient engineering to 19th-century French physiology.
The study of "push" evolved from ancient engineering to 19th-century French physiology.
Humanity has observed osmotic flow since antiquity—notably during the construction of the Egyptian pyramids—but it wasn't scientifically documented until Jean-Antoine Nollet did so in 1748. The term itself was born from the French words endosmose and exosmose, coined by physician René Joachim Henri Dutrochet. He derived them from the Greek word ōsmós, meaning "push" or "impulsion," perfectly capturing the mechanical nature of the movement.
The field moved from observation to precision in 1867 when Moritz Traube invented highly selective precipitation membranes. This allowed scientists to measure osmotic flow accurately for the first time, transforming osmosis from a biological curiosity into a quantifiable branch of physical chemistry.
Modern technology "reverses" osmosis to create drinking water and renewable energy.
Modern technology "reverses" osmosis to create drinking water and renewable energy.
By applying external pressure that exceeds natural osmotic pressure, we can force the process to run backward. This "Reverse Osmosis" (RO) is the global standard for desalination, stripping salt and contaminants from seawater to produce fresh drinking water. While RO requires significant energy to "fight" the natural gradient, a newer field called "Forward Osmosis" is being researched to purify water more efficiently using high-concentration "draw" solutions.
Looking forward, researchers are exploring "Osmotic Power" (or blue energy). By harnessing the pressure difference where freshwater rivers meet the salty sea, we can generate sustainable electricity. Beyond energy, the same principles are being applied to medicine for innovative drug delivery systems that use osmotic pumps to administer medication inside the human body with robotic precision.

The process of osmosis over a semipermeable membrane. The blue dots represent particles driving the osmotic gradient.

The "endosmometer" invented by Dutrochet

Water passing through a semipermeable membrane
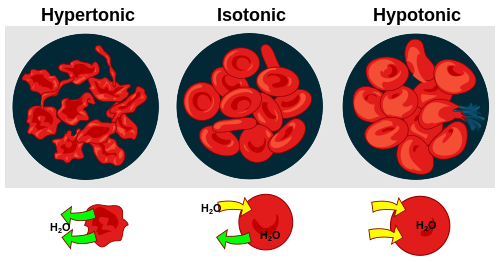
Effect of different solutions on blood cells

Image from Wikipedia
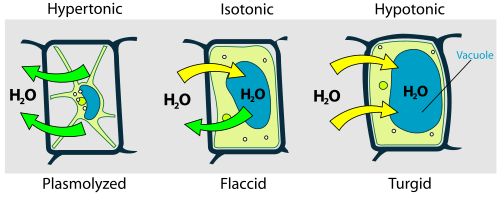
Image from Wikipedia