Catalysis
Catalysts provide an energetic shortcut by lowering activation barriers without being consumed in the journey.
Catalysts provide an energetic shortcut by lowering activation barriers without being consumed in the journey.
A catalyst functions by offering a reaction an alternative mechanism—a "side path" that requires significantly less energy to traverse than the standard route. By stabilizing the transition state of a reaction, the catalyst allows more molecular collisions to result in a successful transformation. Crucially, the catalyst emerges from the process regenerated and unchanged, meaning a tiny amount can facilitate thousands of cycles, measured by the "turnover number" (TON).
This "loop" of interaction and regeneration allows for extreme efficiency. In the decomposition of hydrogen peroxide, for instance, adding manganese dioxide causes immediate, rapid effervescence as oxygen is released. Without the catalyst, the same reaction is so slow that the solution remains stable on a shelf for months. The catalyst doesn't "force" the reaction; it simply removes the kinetic obstacles blocking its natural progression.
Thermodynamics strictly limits catalysts to speeding up existing possibilities rather than creating new ones.
Thermodynamics strictly limits catalysts to speeding up existing possibilities rather than creating new ones.
While a catalyst changes the speed of a reaction, it has zero effect on the final chemical equilibrium. It cannot make a reaction happen that wouldn't eventually happen on its own; it simply helps the system reach its inevitable state faster. This is a fundamental law of thermodynamics—if a catalyst could shift equilibrium, it would effectively be a perpetual motion machine, producing energy from nothing simply by being added and removed.
The catalyst lowers the "kinetic barrier" (the effort to start) but leaves the "thermodynamic barrier" (the energy difference between start and finish) untouched. It speeds up both the forward and reverse reactions equally. If a process is fundamentally non-spontaneous, no amount of catalysis will make it occur without an external energy input like heat or light.
Industrial scale relies on "supported" heterogeneous catalysts to maximize surface area and minimize cost.
Industrial scale relies on "supported" heterogeneous catalysts to maximize surface area and minimize cost.
In the massive chemical plants that produce the world’s fertilizers and fuels, 90% of products involve catalysis. Most of these use "heterogeneous" catalysts—solids acting on liquid or gas reactants. Because the reaction only happens at the "active sites" on the surface of the catalyst, engineers use highly porous "supports" like alumina or activated carbon. These supports act like a structural scaffold, spreading out expensive metals (like platinum or nickel) to expose the maximum possible surface area.
A classic example is the Haber process, which synthesizes ammonia from nitrogen and hydrogen. The triple bond of nitrogen is incredibly strong and difficult to break in the air, but when adsorbed onto the surface of an iron-based catalyst, the bond weakens, allowing the reaction to proceed at industrial speeds. This single catalytic application is responsible for the fertilizers that support nearly half the global population.
The field is evolving beyond heavy metals toward "organocatalysis" that mimics the efficiency of biological life.
The field is evolving beyond heavy metals toward "organocatalysis" that mimics the efficiency of biological life.
Historically, the most powerful catalysts were transition metals, which are often expensive, toxic, or rare. However, the 2021 Nobel Prize in Chemistry highlighted a shift toward "organocatalysis"—using small, metal-free organic molecules like proline to drive reactions. These organic catalysts mimic enzymes, the biological proteins that catalyze every breath and heartbeat in the human body.
Organocatalysts are often cheaper and more environmentally friendly than their metal counterparts. They work through "switchable" or "tandem" mechanisms, where the catalyst can be toggled between states or coupled with others in a single "one-pot" reaction. This move toward "green chemistry" aims to reduce the energy footprint and waste of chemical manufacturing, bringing industrial processes closer to the elegant, low-temperature efficiency of nature.

A range of industrial catalysts in pellet form

An air filter that uses a low-temperature oxidation catalyst to convert carbon monoxide to less toxic carbon dioxide at room temperature. It can also remove formaldehyde from the air.

Generic potential energy diagram showing the effect of a catalyst in a hypothetical exothermic chemical reaction X + Y to give Z. The presence of the catalyst opens a different reaction pathway (shown in red) with lower activation energy. The final result and the overall thermodynamics are the same.

The microporous molecular structure of the zeolite ZSM-5 is exploited in catalysts used in refineries

Zeolites are extruded as pellets for easy handling in catalytic reactors.

Typical vanadium pentoxide catalyst used in sulfuric acid production for an intermediate reaction to convert sulfur dioxide to sulfur trioxide.
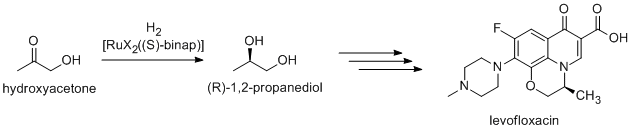
levofloxaxin synthesis